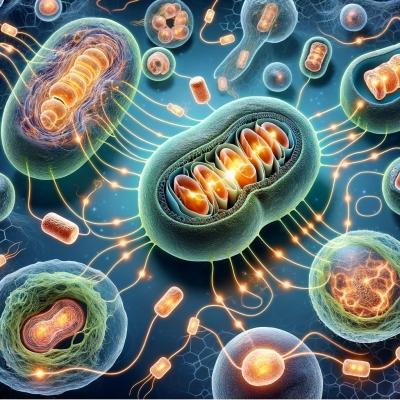
Researchers have found that chemical signals released by mitochondria are communicating to those in other cells. This has an impact on how rapidly organisms age.
Discovery of Mitochondria Communication
In 1993 a discovery upended the idea that aging is due to various breakdowns and failures. Scientists found a mutation in a single gene that doubled a worm’s life span. Later work revealed that genes related to insulin regulation are the vital controllers of aging in many animals, from invertebrates to humans. This suggested that aging is not a random process after all. Instead, there are specific genes that regulate aging. This paved the way for further study into how aging works at a molecular level.
Recently, a set of papers revealed a new biochemical pathway that controls aging: signals passed between mitochondria, which are organelles known as the cell's powerhouse Studying worms, the researchers determined that damage to mitochondria in brain cells produced a repair process that was then amplified, triggering similar reactions throughout the worm’s body. This extended the organism’s life span. The worms with the repaired damage lived 50% longer.
Additionally, scientists discovered that cells that produce eggs and sperm were key to this communication. This finding adds new outlook on fertility issues.
These discoveries add to a other recent studies that suggest that mitochondria can talk to one another even when they are in different tissues. In essence, their function as cellular communication devices throughout the body, which alter the life span of the entire organism. The cell biologist Andrew Dillin found the first hints of this novel pathway about a decade ago. He was searching for life-extending genes in Caenorhabditis elegans worms, and found that genetically damaging the mitochondria allowed the worms to live 50% longer. This was not expected. Dillin had assumed that damaging mitochondria would speed up aging and death rather than extend life, as they are central to cellular function. Yet for some reason, disturbing the smooth functioning of the mitochondria allowed the worms to live longer. Even more surprising: the damaged mitochondria in the worms’ nervous system seemed to be the driver. This showed that mitochondria in some cell types have greater influence. In the last decade, Andrew Dillin has determined the biochemical details of a new pathway that regulates aging, in which mitochondria communicate about cellular health throughout the body.
Tissue-to-Tissue Communication
Dillin and his team have now expanded that finding by discovering new details about how mitochondria in the brain communicate with cells across the worm’s body to boost lifespan. Why was damage to the brain's mitochondria causing this?
The process to generate energy in the mitochondria involves a set of very complex molecules with many different protein parts. When things go wrong, as when some parts are missing or misfolded, mitochondria produce the unfolded protein response, a stress response which delivers repair enzymes to help the proteins assemble correctly. This restores mitochondrial function, keeping the cells healthy. Dillin expected this process to work only inside the neurons with damaged mitochondria, but it triggered cells in other tissues of the worm’s body to turn on repair responses even though their mitochondria were normal. It was this repair activity that helped the worms live longer. What remained mysterious was how this unfolded protein response was communicated to the rest of the organism.
Investigating further, Dillin’s team found that the mitochondria in stressed neurons were using vesicles — bubble-like capsules that move materials around the cell or between cells — to carry a signal called Wnt to other cells in the body. Biologists had known that Wnt helps set up the body pattern during early embryonic development, when it also triggers repair operations like the unfolded protein response. But how could Wnt signaling, when turned on in an adult, avoid triggering the embryonic process?
Dillin guessed that there had to be another signal that Wnt interacted with. Working on this, the researchers found that a gene expressed in the mitochondria of the germline — and in no other cells — can disrupt the developmental program. This indicated that germline cells were critical factors in relaying the Wnt signal between the nervous system and tissues throughout the rest of the body. So, the strength of the germline signal regulates the organism’s life span, Dillin said. As a worm ages, the quality of its eggs or sperm goes down. The decline is also noticed in the germ cells’ lessened ability to transmit signals from the brain’s mitochondria. As the worm grows older, its germline transmission is less effective, and so its body ages.
Source: Quanta Magazine
